Impact on Medical Devices Market
CE Certification Mark
CE certification issued by the Notified Bodies in the EU/EEA countries would remain valid in Switzerland until and unless the applied conformity assessment procedures as per the Swiss MedDO/IvDO are met, and they are issued by a Notified Body that has an equivalent qualification as detailed in Swiss MedDO/IvDO.
Notified Bodies
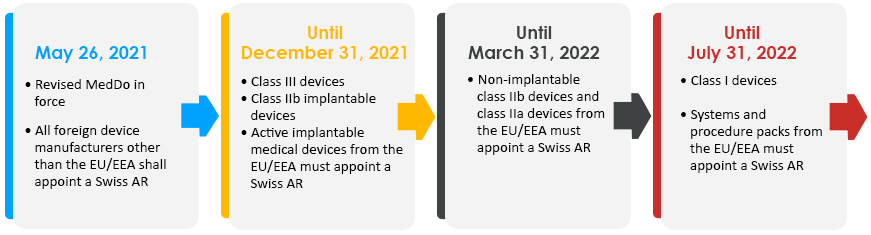
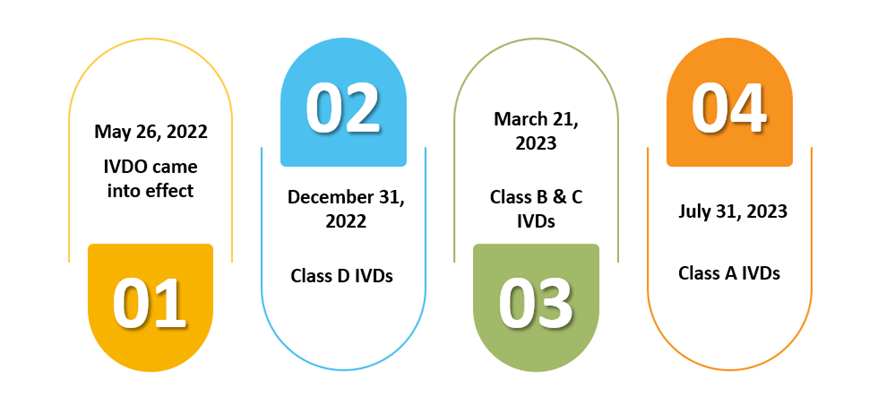
The CE certificates issued under the EU MDD by Swiss Notified Bodies can be considered valid till May 26, 2024, or until the expiration date, whichever comes first.
The CE certificates issued under the EU IVDDD can be considered until May 26, 2025, or until the expiration date (as per the first applicability).
Labeling Requirements
According to the MedDO/IvDO, the name and address of the Swiss AR must be stated on the packaging of the products. If stating the Swiss AR on the product itself, the Instructions for Use (IFU) or documents enclosed with the product are not mandatory.
The following symbol must be used on the packaging, accompanied by the name and address of the Swiss AR. Alternatively, the terms ‘CH Authorized Representative,’ ‘CH-REP,’ or ‘Authorized Representative for Switzerland’ can be used instead of the symbol.